VARIEGATED CUTWORM LIFE CYCLE AND HABITS
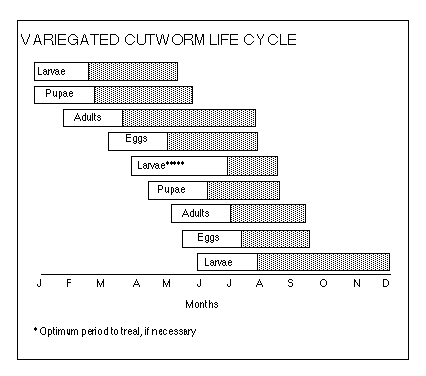
Variegated cutworm, Peridroma saucia, is a common pest of many
vegetable and field crops, including mint. They overwinter as half grown larvae
in soil or under plant debris in or around mint fields, begin feeding in April, and
mature in late April and May. Larvae pupate in earthen cells in the soil. Adults
emerge in May and early June and deposit eggs in clusters of 200 to 500 on the
undersides of leaves. Eggs hatch in 4 to 7 days and larvae begin feeding on
plant foliage. Larvae feed for 4 to 6 weeks and then pupate in the soil. Summer
generation adults emerge in late August and deposit eggs. Larvae hatching
from these eggs feed until cold weather and then become inactive and
overwinter. There are two overlapping generations each year. In IPMP, there is
a variegated cutworm development model, and a variegated cutworm economic
threshold program. See also variegated cutworm management and control.
 Often the larvae of other species occur on and defoliate mint at about the same
time as the variegated cutworm. Bertha armyworm, Memestra configurata, is
often seen in mint fields, particularly west of the Cascade Mountains. Its life
cycle is about the same as that of the variegated cutworm. However, at times
Bertha armyworm larvae may be present before detectable populations of
variegated cutworm. Because its leaf feeding damage usually occurs earlier in
the growth of mint and its population density is generally less than that of
variegated cutworm, it is important to distinguish between the two species. An
early insecticide application against this species may not necessarily control
variegated cutworm. One well-timed remedial application for cutworms and
loopers reduces cost and pesticide load on the field, and minimizes other
possible problems, such as the induction of spider mite problems, destruction of
beneficial insects, and increased pressure for resistance development in pest
insects.
Often the larvae of other species occur on and defoliate mint at about the same
time as the variegated cutworm. Bertha armyworm, Memestra configurata, is
often seen in mint fields, particularly west of the Cascade Mountains. Its life
cycle is about the same as that of the variegated cutworm. However, at times
Bertha armyworm larvae may be present before detectable populations of
variegated cutworm. Because its leaf feeding damage usually occurs earlier in
the growth of mint and its population density is generally less than that of
variegated cutworm, it is important to distinguish between the two species. An
early insecticide application against this species may not necessarily control
variegated cutworm. One well-timed remedial application for cutworms and
loopers reduces cost and pesticide load on the field, and minimizes other
possible problems, such as the induction of spider mite problems, destruction of
beneficial insects, and increased pressure for resistance development in pest
insects.
MANAGEMENT AND CONTROL OF FOLIAGE FEEDING
CUTWORMS
Foliage feeding cutworms and armyworms include the variegated cutworm
and other foliage-feeding cutworms such as spotted cutworm, Amathes c-
nigrum, western yellowstriped armyworm, Spodoptera praefica, and the Bertha
armyworm. These different cutworm species may occur together in the same
field during July and early August. The sampling program and treatment
threshold described below for the variegated cutworm may also be used for the
aggregate of these species.
For variegated cutworm, inspect fields closely from mid-June to just prior to
harvest, remembering that if an insecticide application is considered, the
preharvest interval must be observed. Growers may want to consider
harvesting earlier to avoid further crop injury. Sweep net samples can be used
to sample small larvae (first, second, and third instars). Usually 10 straight line
sweeps at 5 different sites in fields up to 30 acres are sufficient to evaluate
larval populations. Add an additional site for every additional 10 acres. Largest
collections of these smaller larvae will occur on cool, overcast days, or when
fields are sampled early in the morning or near dusk on still days. Avoid sweep
net sampling when mint is water-stressed or foliage is wet. Very often, more
than 50 per cent of the cutworms found in samples will be parasitized; this may
alter the treatment thresholds.
The decision to apply an insecticide is usually based on the average number of
larvae found per 1,000 sq cm (cm2)(an area slightly larger than 1 sq ft) on the
soil surface. To estimate larval populations of fourth, fifth, and sixth instars,
inspect the soil surface by first vigorously shaking mint foliage and closely
observing and recording the number of larvae per 1000 cm2 randomly through
the field. Take a ground search sample every 5 acres for fields up to 30 acres.
Add an additional site for every 10 acres in fields that exceed 30 acres. Look
very closely for small and curled-up larvae under and in folded leaves on the
ground. Remember that larvae can fall into cracks on the soil surface. When
leaf-chewing is quite evident and cutworm counts from ground searches are
low, consider returning after dark and sampling with a sweep net when the
larvae actively feed on the foliage.
Sequential sampling plans have been developed for variegated cutworm using
sweep net samples to estimate larvae (instars 2 to 4) and for ground search
samples (1000 cm2) to estimate larval instars 4 to 6 (Coop, 1987). Using these
plans, treatment of larval instars 2 to 4, sampled with a sweep net, is
recommended if 60 larvae are collected from a minimum of 11 different field
sites (a minimum of 10 sweep net samples should be taken at each site).
Treatment is not recommended if fewer than 44 larvae are collected in sweep
net samples. For ground search sampling, treatment of larval instars 4 to 6 is
recommended if 24 larvae are collected in 1000 cm2 samples taken from a
minimum of 18 different sites. Treatment is not recommended if fewer than 17
larvae are collected in the 1000 cm2 samples.
A sex pheromone is commercially available and can be used to detect and
monitor adult males of the variegated cutworm in the spring. Trapping males
could provide valuable early season information to growers concerning the
potential need to control cutworm larvae during June and July (Coop, 1987).
Sticky traps baited with this lure can be set in fields in late April and monitored
weekly or biweekly through June. Although action levels for an insecticide or Bt
treatment have not been developed based on moth catches, it is likely that large
and continual catches greater than 25 per week will result in similarly large
populations of larvae being observed approximately 2 weeks following peak
trap counts. The real value of pheromone traps lies in the fact that they signal
when to begin inspecting fields for larvae, thereby greatly improving timing of an
insecticide application, if one is necessary. Also be aware that, occasionally,
large trap catches will not result in large larval populations. This is particularly
true when the mint field does not have a resident population of variegated
cutworm and the trap catches are a result of males being lured into traps from
other crops. Conversely, a small trap catch does not necessarily mean that an
action level will not be exceeded by the larvae.
# K+ Biological Control by parasitoids
Naturally occurring predators and parasites play an important role in
suppressing cutworm populations throughout all mint-growing areas in Oregon.
The percentage of parasitism may reach 80 to 90 per cent in some fields. The
principal parasites of cutworms and loopers on mint are Meteorus communis,
Nepiera sp., Campoletis sp., and Copidosoma sp. (Coop, 1987; Coop and
Berry, 1986). Growers and consultants are urged to consider that it is likely that
a significant proportion of the larvae in samples may be parasitized. Leaf
consumption by parasitized larvae is much reduced and there is very little
reduction in oil yield caused by these larvae (Coop and Berry, 1986).
Parasitized larvae can be distinguished from nonparasitized larvae only by
dissection. Select some of the largest larvae from the sweep or ground search
sample. With each, cut the head off, and pull the larva apart. If the larva is
parasitized, another smaller larva of the pasrasite will be found inside the
cutworm larva. If time permits, larvae can be reared in the laboratory to
determine the number parasitized. Depending on the percentage of larvae
parasitized, you can increase the suggested action thresholds.
If treatment is justified, refer to the insecticide table for a list of insecticides and
rates that are registered for use on mint to control cutworms. Orthene generally
has been more effective in controlling larger cutworm and looper larvae and
grasshoppers. Lannate controls smaller larvae and has been shown to prevent
egg hatch in laboratory studies and field observations in other crops. Lannate
also satisfactorily controls populations of adult mint flea beetle when present at
the time of application -- Orthene does not. Research has shown that
variegated cutworm feeding on peppermint is less susceptible to insecticides
because the terpenes found in mint leaves induce enzymes that detoxify the
insecticides (Berry et al., 1980). On the other hand, certain peppermint
terpenes have been shown to increase mortality of variegated cutworm larvae
and pupae (Harwood, 1988; Harwood et al., 1990).
# K Bacillus thuringiensis (Bt) applied at 1 to 2 quarts/acre can reduce
populations of small cutworms. It benefits from the addition of a spreader sticker
or wetting agent and is more effective if applied at night, when larvae are
actively feeding on foliage being sprayed, and in the absence of direct sunlight
and extreme temperatures. It does not control pests other than loopers,
cutworms, and armyworms. Activity on variegated cutworm and other cutworms
and armyworms is variable, depending on the trade product and its formulation.
The major disadvantage of the use of Bt is the fact that the dense mint foliage
prevents penetration and coverage of the leaves with Bt.
Entomopathogenic nematodes have recently been tested to provide control of
variegated cutworm in peppermint (Berry et al. 1993).
 Often the larvae of other species occur on and defoliate mint at about the same
time as the variegated cutworm. Bertha armyworm, Memestra configurata, is
often seen in mint fields, particularly west of the Cascade Mountains. Its life
cycle is about the same as that of the variegated cutworm. However, at times
Bertha armyworm larvae may be present before detectable populations of
variegated cutworm. Because its leaf feeding damage usually occurs earlier in
the growth of mint and its population density is generally less than that of
variegated cutworm, it is important to distinguish between the two species. An
early insecticide application against this species may not necessarily control
variegated cutworm. One well-timed remedial application for cutworms and
loopers reduces cost and pesticide load on the field, and minimizes other
possible problems, such as the induction of spider mite problems, destruction of
beneficial insects, and increased pressure for resistance development in pest
insects.
Often the larvae of other species occur on and defoliate mint at about the same
time as the variegated cutworm. Bertha armyworm, Memestra configurata, is
often seen in mint fields, particularly west of the Cascade Mountains. Its life
cycle is about the same as that of the variegated cutworm. However, at times
Bertha armyworm larvae may be present before detectable populations of
variegated cutworm. Because its leaf feeding damage usually occurs earlier in
the growth of mint and its population density is generally less than that of
variegated cutworm, it is important to distinguish between the two species. An
early insecticide application against this species may not necessarily control
variegated cutworm. One well-timed remedial application for cutworms and
loopers reduces cost and pesticide load on the field, and minimizes other
possible problems, such as the induction of spider mite problems, destruction of
beneficial insects, and increased pressure for resistance development in pest
insects.